A Breath Test for Diagnosing Lung Infection in 15 Minutes
CAMBRIDGE, MA—When the Infectious Diseases Society of America released its new guidelines for aspergillosis, they admitted “critical gaps in knowledge remain” regarding tools for early detection, evaluation of response and therapies. That’s tough news for the more than 10 million worldwide at risk of developing the lung condition, to say nothing of the nearly 40,000 admitted to hospitals in the U.S. for it every year. The cost for diagnosing invasive aspergillosis (IA)—the most deadly form of this condition—is also rising and currently tops $550 million annually.
Now Draper is developing a breath-based test for invasive aspergillosis in collaboration with Brigham and Women’s Hospital in Boston. The breath capture and analysis system is producing favorable results during initial clinical testing at BWH, a promising development for clinicians who want to shorten the time-to-diagnosis for IA. IA is a leading cause of death in patients with compromised immunity.
https://www.youtube.com/embed/16uuZuYC-c0
(Courtesy CBS Boston)
“It is challenging to differentiate between fungal, bacterial and viral lung infections. Protocols to diagnose invasive aspergillosis today may take well over a week. That’s not good news for patients whose rate of survival drops with every day’s delay in diagnosis,” said Kenneth Markoski, a Distinguished Member of Draper’s Technical Staff.
Markoski is working collaboratively with Sophia Koo, MD, a physician in BWH’s Division of Infectious Diseases who is piloting the device in a clinical setting. “Current diagnostics for lung infections, including fungal, bacterial and viral pneumonia, are limited,” said Koo. “We hope that this rapid, noninvasive approach to the identification of the underlying pneumonia pathogen can bring some precision to how we prescribe antifungal drugs and antibiotics, and help us determine whether these pathogens are being treated effectively by these drugs.”
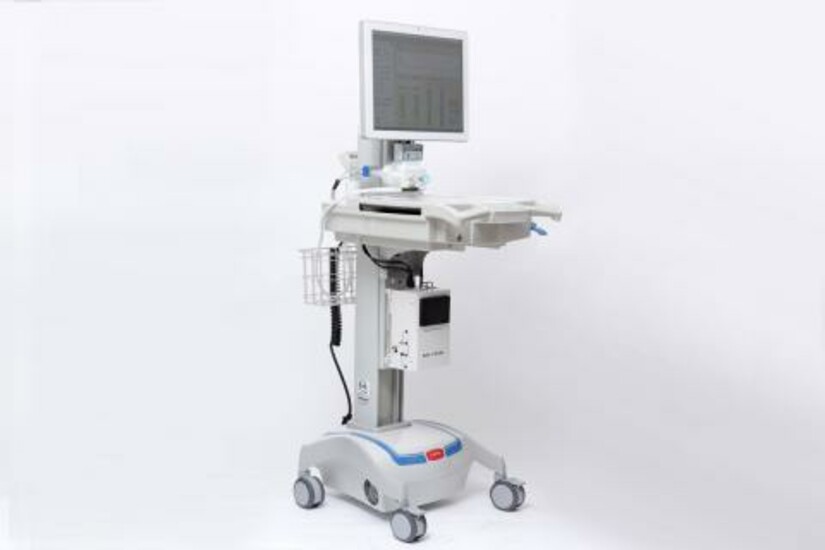
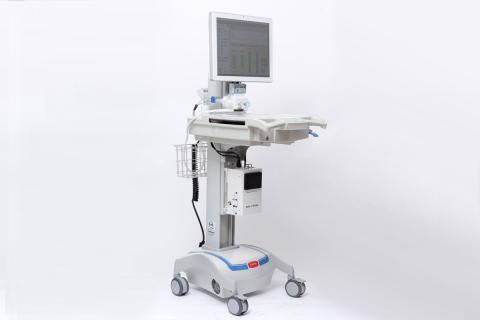
Markoski and his colleagues developed a novel device called the microAnalyzer™ that uses a differential mobility spectrometry (DMS) sensor, coupled with other technologies, to identify biomarkers in a patient’s breath samples that are indicative of disease or ailments. These biomarkers often may be detected by the system prior to the presence of symptoms. “With Draper’s microAnalyzer, the physician may begin treatment immediately upon receiving the system’s response, reducing or eliminating the need for invasive lung biopsies and multi-day lab cultures,” Markoski said.
Draper’s microAnalyzer is a portable device that can fit on a hospital cart for immediate access at the point-of-care. Smaller, less complex and less expensive than a mass spectrometer, the microAnalyzer performs similar high-quality chemical measurements immediately. The device, which couples DMS technology with a preconcentrator and a miniature fast gas chromatography column, features a tunable ion filter that can be set to reject interferents and select biomarkers of interest. The result is that the microAnalyzer can detect trace vapors as low as a few parts per trillion.
A major achievement for Markoski and his team was to design the microAnalyzer so that it can be programmed to detect other biomarkers simply by running a different computer file. Unlike other breath-based detectors, Draper’s microAnalyzer does not require hardware modifications, such as coatings and wavelength changes, to detect new compounds of interest. This breath analysis platform technology will be used for the detection of ailments such as other infectious diseases and cancers.
According to Anthony Coston, Draper’s Assistant Director of Biomedical Solutions, breath sampling at the bedside could bring benefits beyond patient care. “Antifungal drugs are toxic, costly and have numerous complicated interactions with other medications. By developing a device for the rapid, accurate, noninvasive identification of IA, we can improve clinical outcomes and guide efforts to prescribe antifungal therapy more precisely to patients who require treatment and spare those who do not.”
Released December 14, 2017