Draper Device Could Accelerate Restoration of Hearing
Device is ready for use by commercial partners
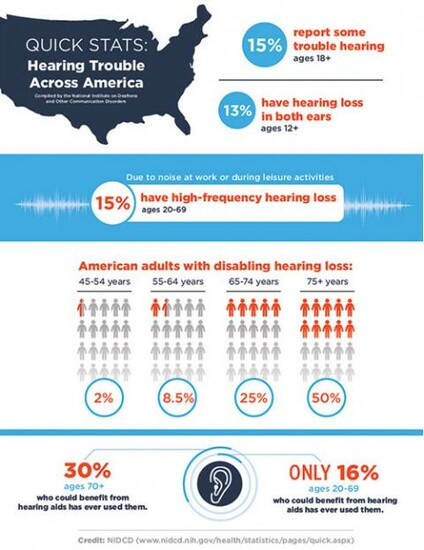
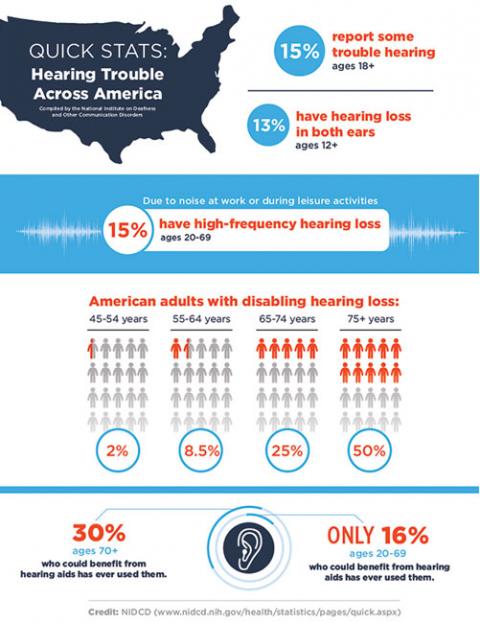
CAMBRIDGE, MA – Approximately 15 percent of U.S. adults suffer from hearing loss; its impact can be devastating. In addition to trouble communicating and enjoying daily life, those who deal with the condition commonly report difficulty focusing and making decisions, anxiety that people are talking behind their back, and loss of self-esteem. While researchers are developing drugs potentially capable of treating hearing loss, they often encounter difficulty demonstrating preclinical efficacy due to uncertainty regarding concentration of the medication at the intended delivery site to prove that it is responsible for observed changes in hearing.
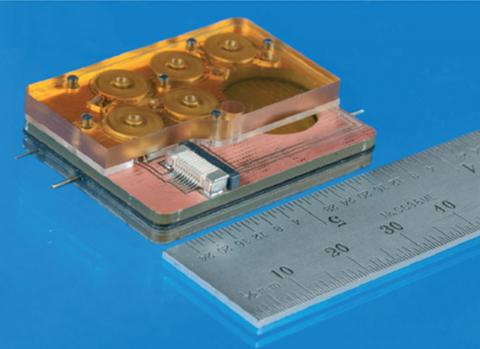
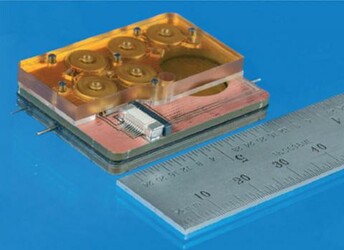
Draper engineers, in collaboration with clinicians and scientists at Massachusetts Eye and Ear, have developed a device capable of delivering drugs directly to the cochlea—an area of the inner ear difficult to reach with injections and systemic drug delivery—that also can be used to sample drug concentration in the cochlea, a key step toward securing FDA approval.
“Hearing loss drugs hold the potential to restore the natural sense of hearing, but preclinical measurements of drug concentrations in the inner ear will be critical in demonstrating that the drug is responsible for observed hearing changes prior to human trials,” said Jeff Borenstein, Draper’s principal investigator for the intracochlear drug delivery device.
Other approaches generally deliver drugs to treat hearing loss through the middle ear, which relies on indirect transport to the inner ear and may require repeat injections. Draper’s device, developed with funding from the NIH through its National Institute on Deafness and Other Communication Disorders (NIDCD), builds on the company’s expertise in precision instrumentation and microsystems in concert with Mass. Eye and Ear’s world-leading expertise in the molecular biology and clinical aspects of hearing loss. The device is capable of delivering one or more drugs in an automated, timed sequence directly into the fluid of the inner ear. Draper’s device also features a reciprocating delivery cycle that keeps the volume of inner ear fluid constant while mixing in the drug, which is intended to increase the treatment’s safety and efficacy.
“Ultimately, this device should be useful for delivering drugs where and when they are needed, in a concentration where they can be effective, and without unwanted systemic side effects,” said Sharon Kujawa, director of audiology research at Mass. Eye and Ear. “Some of the most promising future treatments may require the delivery of combinations of drugs in a timed and sequenced manner to be effective, and these requirements will not easily be met with systemic delivery. With the local, intracochlear delivery provided by this device, it should be possible to take advantage of present and future breakthroughs in hearing loss treatment and prevention with the potential to benefit our patients with hearing loss.”
During recent testing designed and executed in collaboration with Mass. Eye and Ear, Draper replaced the commercially available actuator used in earlier tests with custom designed hardware that is more precise and smaller, making it simpler to implement in animal models. Borenstein and his Mass. Eye and Ear colleagues including Kujawa published results of their recent work in the peer-reviewed journal Lab on a Chip.
In addition to improving confidence in results from preclinical trials, a wearable version of the device could be available in the near future to treat patients who experience hearing loss as a side effect to medications like cisplatin, a chemotherapy drug, or tobramycin, which is used to treat cystic fibrosis, Borenstein said.
Released January 26, 2016