Engineers Develop 3D-Printed Microfluidics Device to Personalize Immunotherapy in the Clinic
CAMBRIDGE, MA—Cancer cells can often evade the immune system, a situation that presents a challenge to patients, physicians and immunotherapy developers. Ordinarily, to identify self from “non-self,” the immune system uses “checkpoints”—molecules on certain immune cells that need to be activated (or inactivated) to start an immune response. Except that sometimes cancer cells find ways to use these checkpoints to avoid being attacked.
The recent discovery of immune checkpoint inhibitors (ICIs)—compounds capable of blocking a tumor’s ability to evade attack by a patient’s immune system—is showing tremendous promise in improving patient outcomes for many cancers, a breakthrough resulting in last year’s Nobel Prize in Medicine. However, in order to realize the full potential of this new therapeutic approach, new tools are needed to probe the underlying mechanisms and to better predict the efficacy of emerging compounds for particular patients and cancer types.
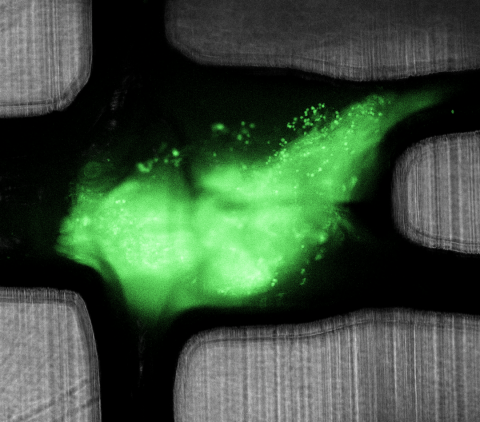
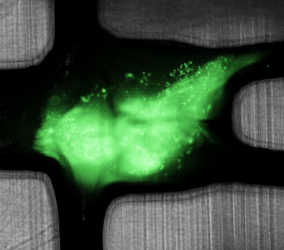
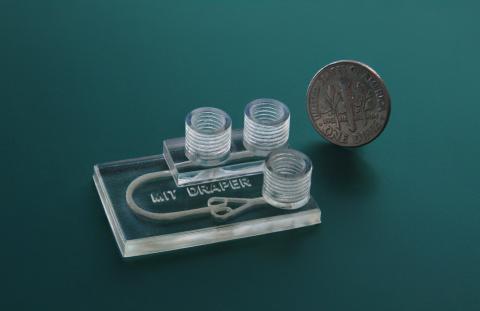
Recently, engineers at Draper demonstrated a laboratory method for testing how well these ICIs work in disrupting the immunoinhibitory behavior of a tumor tissue. They used a device about the size of a quarter that can sustain the viability of biopsied tissue fragments under dynamic microfluidic perfusion for at least 72 hours while drug treatments are administered and tested for efficacy.
The results appeared online May 6 in the journal Advanced Healthcare Materials. Authors include Ashley Beckwith, a Draper Fellow and Ph.D. candidate at MIT; Jeff Borenstein, Principal Investigator for the Immuno-Oncology research program at Draper; and Luis F. Velásquez-García, Principal Research Scientist and leader of the Micro- and Nano-enabled Multiplexed Scaled-down Systems Group at MIT.
Beckwith led the design and development of the device under the direction of Draper and MIT, and used novel fabrication techniques to manufacture it, including a 3D printer and a commercially available printable resin. “Being able to 3D-print a microfluidic platform that can facilitate biomedical analyses enables us to overcome barriers in design and fabrication of such devices without compromising system capability. Through the successful implementation of this printed device to address relevant medical needs, we hope to demonstrate the potential for additive manufacturing to advance the field of microfluidics,” Beckwith said.
According to the authors, the paper is the first report of human tumor fragments cultured in a dynamic perfusion system capable of testing the effect of the administration of ICIs on resident tumor-infiltrating lymphocytes. The engineers used the device to develop a new methodology for modeling and analyzing tumor response for the improved prediction of patient-specific immunotherapy efficacy.
With this first-of-its-kind device, clinicians move a step closer to being able to personalize immunotherapy at the clinical level, according to Borenstein. “ICIs have opened a tremendously promising new avenue for treating cancer, but response rates for different patients and types of cancer are difficult to predict. This new technology has the potential to accelerate the development of new ICI therapies, and ultimately to accurately predict ICI efficacy before administration to a patient,” he said.
The paper “Microfluidic Model for Evaluation of Immune Checkpoint Inhibitors in Human Tumors” is available at the journal’s website.
Released June 6, 2019