Sense of Touch, Awareness Within Reach for Lower Arm Amputees
CAMBRIDGE, MA – Nearly two million Americans have lost a limb. Those who have lost a portion of an arm may have a prosthesis that helps them get through daily life, but activities like driving and playing sports are difficult without a natural sense of feeling and awareness.
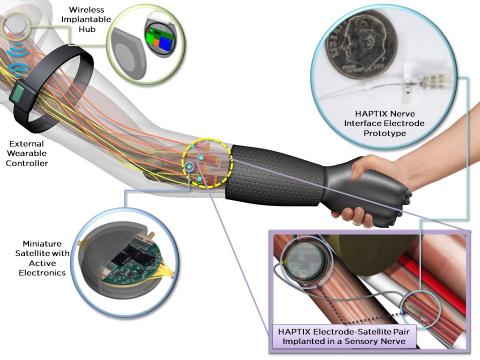
Draper is developing technology intended to help patients regain both a realistic feeling of touch as well as what is referred to as ‘proprioception’ – the ability to process and integrate limb orientation information that, for example, allows you to touch your finger to your nose even when your eyes are closed. The development of advanced miniaturized implantable devices allowing proprioceptive feedback will enable more intuitive and dexterous control of prosthetic arms.
Draper collaborated with Nerves Incorporated and University of Texas Southwestern (UTSW) Medical Center scientists and clinicians to demonstrate early feasibility data to control limb movement in animal studies. The team used a novel interfacing approach that employed miniaturized electrodes fabricated from advanced manufacturing techniques to record sensory and motor signals from individual nerve fibers of interest.
The implantable device is intended to provide precision neurostimulation therapy to individual nerve fibers, enabling amputees using prosthetic hands to feel several gradations of sensory feedback. Detecting motor nerve signals will also help amputees with complex hand grasping tasks such as gently holding a child’s hand. “This closed-loop approach will empower the amputee with intuitive feedback from their prosthesis, which limb-replacing implants on the market today don’t offer,” said Philip Parks, Draper’s HAPTIX program manager.
“There’s not a lot of space in the arm for an implant, so our experience building highly capable microsystems for our customers, integrated with our expertise in advanced algorithm development, can be beneficial in helping restore feeling to those with prosthetic hands,” said John Lachapelle, Draper’s principal investigator for HAPTIX. This study is funded by the Defense Advanced Research Projects Agency’s Hand Proprioception and Touch Interfaces (HAPTIX) program.
Following continued technology development, Draper will begin integration of low power microchips and other miniaturized wireless modules to complete the development of the HAPTIX sensory feedback system. This integrated system will go through rigorous testing according to FDA standards prior to clinical trials in patients, which are planned to commence in fall 2016. If the clinical trials are successful, the device could be available to patients within four years.
In addition to the surgeons and neuroscientists from UTSW and Nerves Incorporated, who are testing the engineered devices and providing feedback, Draper’s interdisciplinary HAPTIX team includes Boston Scientific, which is contributing neurostimulation experience towards the development of reliable device packaging, and Bryan McLaughlin of Micro-Leads, who is contributing medical electronics system design expertise.
Many critical components for this project, including customized microelectronics and software algorithms to receive, process, and stimulate sensation have been matured using government funds and internal investments. For DARPA’s SUBNETS program, which supports the president’s BRAIN initiative, Draper is working with clinical partners at Massachusetts General Hospital to develop an implantable deep brain stimulation device to provide closed-loop treatment of neurological disorders like traumatic brain injury, as well as psychiatric conditions like PTSD, depression, and anxiety. Leveraging knowledge from SUBNETS and HAPTIX, Draper is also working with a Fortune 500 companies to developing advanced neuromodulation systems to treat autonomic nerve diseases, with clinical trials expected in late 2016.
Released October 19, 2015