U.S. Dept. of Health and Human Services Taps Draper to Develop Lung-on-a-Chip Technology to Study SARS-CoV-2
CAMBRIDGE, MA—Draper has received a $719,000 federal contract from the U.S. Department of Health and Human Services (HHS) to enhance the company’s tissue-chip platform for advanced studies of viral infection. With the contract, Draper will design a model for human airway tissue and adapt its PREDICT96 platform to collect data for SARS-CoV-2 infection studies. The 18-month performance period begins in September.
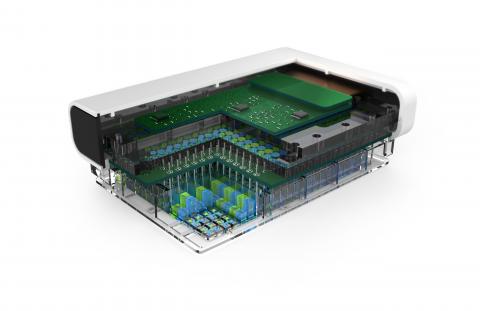
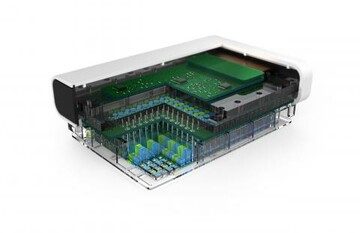
The funding is from the Biomedical Advanced Research and Development Authority (BARDA) which is a part of the HHS Office of the Assistant Secretary for Preparedness Response, and in collaboration with the National Center for Advancing Translational Sciences (NCATS). PREDICT96 is a high-throughput, 3D tissue culture platform.
The study of respiratory health, diseases and treatments is typically done through animal studies or culture models using animal or human cells. Draper has advanced the science by developing a 3D tissue culture platform and specific organ tissue models on chips designed to accelerate drug discovery, toxicity screening and efficacy testing.
“When the HHS announced its new ImmuneChip+ program in March—specifically seeking proposals to generate models of immunity on a chip—we were fortunate to be in a strong position to apply,” said Joe Charest, head of strategy and business development, pharmaceutical R&D technologies, at Draper.
BARDA’s ImmuneChip+ program requires that all applicants already have a mature, validated tissue chip, in this case the lung. With the funding, awarded through BARDA’s Division of Research, Innovation, and Ventures (DRIVe), Draper is on contract to develop an ImmuneChip+ based on its lung-on-chip, combining human tissue with an immune system component in a single platform that can be machine-manufactured and that includes multiple in-line sensors for long-term tissue monitoring.
The Draper team will be working not only to characterize how coronavirus and other infections affect the lungs but to evaluate the chip’s usefulness in developing therapies for related respiratory pathologies, as well. The lung-on-chip can be used to model healthy and diseased tissue, measuring immune system activity and response to drugs or toxins.
To develop an organ-on-a-chip, human cells are used to manufacture 3D organ-like structures. These small structures mimic the function of organs such as the liver, kidney, gut and, in this case, the lungs.
Once generated, these structures are placed on PREDICT96, a device equipped with 96 individual wells and 192 integrated pumps that circulate fluid through a system of fluid channels; sensors monitor individual organs. A blood substitute keeps lung-on-chip tissues alive and is used to circulate compounds and therapies of interest to these artificial organs. Channels guide these agents to tissues, with sensors measuring temperature, pH, oxygen levels and other metrics in real time.
The contract calls for development of a custom-engineered 96-device platform called PREDICT96-AIR that can be used to grow and test human primary airway epithelial cells. The PREDICT96 platform is designed to integrate with pharmaceutical infrastructure, making 3D human tissue models a readily available tool for drug discovery, toxicity screening and efficacy testing.
“We look forward to joining BARDA to explore alternatives to in vivo testing to speed the discovery and development of new treatments,” said Jeff Borenstein, a biomedical engineer and Laboratory Fellow at Draper.
This project has been funded in whole or in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, under Contract No. 75A50121C00076.
Released September 23, 2021