New Device Could Be a Safer Alternative to Ventilators for Severe Lung Injury
CAMBRIDGE, Mass.—Draper, an engineering innovation company, has announced that it has entered into a partnership with the Biomedical Advanced Research and Development Authority’s (BARDA) Division of Research, Innovation and Ventures (DRIVe) to develop a groundbreaking treatment for acute respiratory distress syndrome (ARDS). BARDA is a component of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services.
ARDS is a serious medical condition that can be caused by infections, exposure to toxic radiation or chemicals, smoke inhalation, lung injury or trauma. Approximately 200,000 Americans are diagnosed annually with ARDS.
Draper’s innovative blood oxygenation technology, BLOx, is a new alternative to current mechanical ventilation (MV) and a revolutionary advance in extracorporeal membrane oxygenation (ECMO) methods. The company’s goal is to improve ECMO safety and portability for better patient outcomes and to enable extended operation of ECMO devices.
“Draper is proud to develop the next generation of ECMO to improve health outcomes and access for critically ill patients,” said Jonathan Cash, vice president and general manager of Biotechnology at Draper. “This is a wonderful example of Draper’s commitment to solving our nation’s most challenging problems on behalf of current and future generations.”
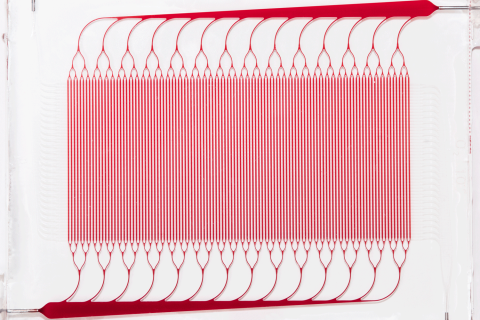
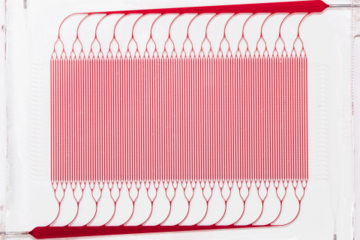
BLOx is a bioinspired microfluidic blood oxygenator built using fully 3D branching networks of microchannels designed on the basis of principles drawn from the human body’s microcirculation. Unlike current commercial devices, BLOx is specifically designed to reduce reliance on anticoagulant therapy, such as heparin. This will reduce the healthcare burden of ARDS by offering a simplified approach with lower risk, providing a cost-efficient alternative.
Current commercial ECMO devices are based on hollow fiber membrane oxygenators (HFMO), which meet requirements for oxygen transfer but suffer from significant shortfalls in management of clotting, bleeding and inflammatory responses. Draper’s BLOx seeks to overcome many of these shortcomings by more closely mimicking the nature of human blood flow using a 3D branching architecture for microchannels that carefully manages the blood shear and other key fluid dynamic parameters within a narrow range. This reduces the need for coagulation mitigation procedures such as complex systemic heparin dosing protocols and nitric oxide administration during operation.
Recently, several studies revealed an increase in ECMO as a treatment option for ARDS. One study reported a spike in the number of adult ECMO cases after the H1N1 pandemic. Another study revealed an increase in ECMO as a treatment for ARDS over the last 15 years due in part to improvements that made ECMO devices more biocompatible and compact.
“Draper’s work under this BARDA contract will lay the foundation for devices that can be used in the prolonged field care setting, in addition to critical care settings where this type of technology can be applied to other diagnoses such as kidney disease. Advances in this field have the potential to be lifesaving for those affected by ARDS who are not candidates for MV or alternative treatments,” said Jeffrey Borenstein, Ph.D., Laboratory Fellow at Draper and principal investigator of the BLOx device.
The partnership with BARDA’s DRIVe Healing Lungs program is a significant milestone in Draper’s mission to address the huge unmet need for treatment of respiratory failure and transform the standard of care for patients suffering from ARDS.
This project has been supported in whole or in part with federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA), under contract number 75A50123C00030.
Released January 23, 2024