Draper Develops Artificial Lung Technology with $4.9M U.S. Army Grant, Earns ASAIO Award
CAMBRIDGE, MA—Artificial lung assist devices have been called life-savers for people who suffer respiratory failure. But such devices carry potential risks, including blood clots, bleeding and sepsis. The answer for improving a device’s function while reducing side effects may lie in engineering it so that it more closely mimics the human lung.
Draper has done just that, making advances in an artificial lung technology called extracorporeal membrane oxygenation, or ECMO. During ECMO, blood is drawn from the patient’s vascular system and circulated outside the body by a mechanical pump through an oxygenator and heat exchanger. Carbon dioxide (CO2) is removed and oxygen-saturated blood is returned to the body.
In spite of decades of advances, ECMO still suffers from limitations. The device can become clogged with blood clots, which diminishes the membrane’s ability to transfer oxygen and CO2. ECMO can experience inconsistent blood flow and pressure across the device, and require high levels of anticoagulant, which can cause bleeding problems. Experts agree that reducing complications will improve outcomes for ECMO patients.
To develop its artificial lung technology, Draper tapped into biomimetics, an interdisciplinary field in which principles from engineering, chemistry and biology are applied to the synthesis of materials, synthetic systems and machines that have functions that mimic biological processes.
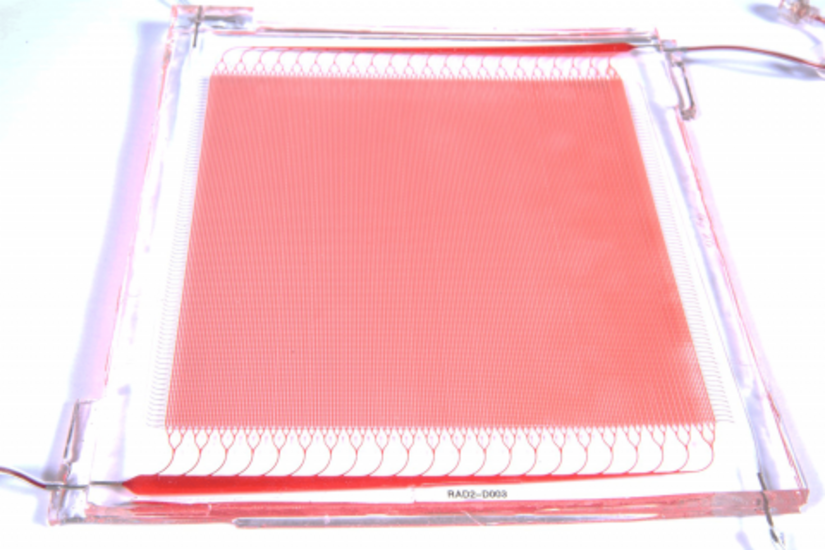
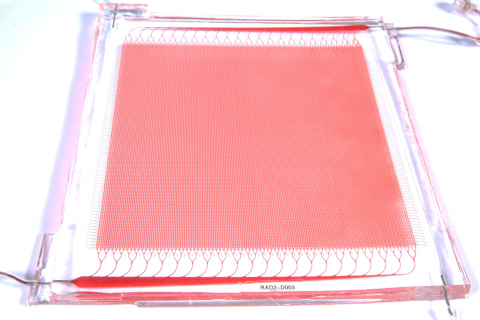
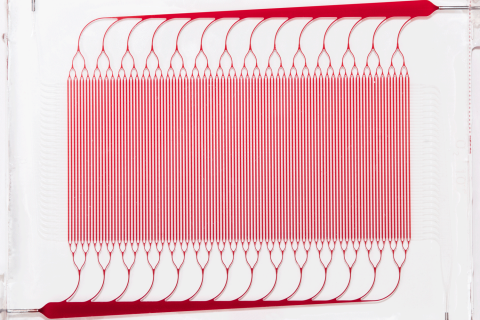
Draper fabricated silicone layers, patterned with either blood channel or gas channel networks, and bonded them together with an intervening thin gas transfer membrane. The multiple sandwich structures were stacked into a three-dimensional network joined by biomimetic blood distribution manifolds to produce a fully 3D, physiologically inspired blood circuit.
Draper researchers tested the multilayer, microfluidic blood oxygenator at blood flow rates approaching clinically relevant levels. They found that a device coated with anticoagulant significantly decreased thrombus accumulation, and helped to stabilize blood pressure, compared to a device without the coating. In an upcoming paper, Draper reports their oxygenator operated at blood flow rates up to 400 milliliters per minute, the highest ever achieved in a complex microfluidic device.
Overall, Draper’s device maintained critical dimensions such as gas transfer membrane thickness and blood channel geometries, and controlled levels of fluid shear within narrow ranges throughout the cartridge. These design features allowed the device to improve upon a nagging performance issue with ECMO, where contact of blood proteins with artificial surfaces, such as the membrane of the oxygenator or tubing, can cause blood clotting.
Engineer Joe Santos helped design the device, called BLOx. “There is a critical need for low prime-volume, lower flow respiratory support devices. There is also an urgent requirement for simpler, safer and more portable ECMO technologies for treatment of injuries in remote locations, such as battlefields and natural disaster sites. Draper’s technology can transform the way ECMO is being done today,” Santos said.
Jeff Borenstein has been working on biomimetic microfluidic oxygenators for more than a decade at Draper. “We believe microfluidic oxygenators have emerged as a potential promising avenue for improving the efficiency and safety of ECMO. Despite wide use, ECMO is limited in its accessibility because it is extremely complex and difficult to administer. We think our approach is simpler and safer, and will lead to wider use,” Borenstein said.
The American Society for Artificial Internal Organs recently recognized the microfluidic blood oxygenator research by naming it the Top Pulmonary Abstract of 2021.
This work was funded by the U.S. Army Medical Research Acquisition Activity and supported by the U.S. Army through the Peer Reviewed Medical Research Program under award number W81XWH1910518. Draper’s four-year, $4.9 million award is scheduled to run through July 2022. Opinions, interpretations, conclusions, and recommendations are those of the author, and are not necessarily endorsed by the U.S. Army Medical Research Acquisition Activity.
Released July 29, 2021