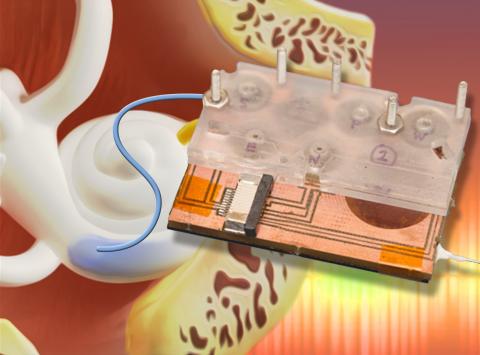
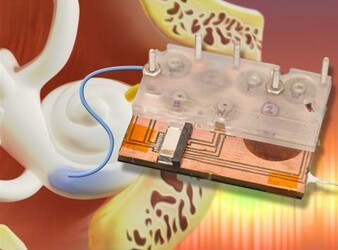
Draper Device Could Boost Confidence of Pre-Clinical Trial Results For Hearing Loss Treatment
CAMBRIDGE, MA – More than 360 million people world-wide suffer from hearing loss, according to the World Health Organization, a disability that can negatively affect everyday life, contributing to social isolation and frustration for adults and speech and educational delays for children.
Today, researchers are developing drugs that would be delivered to the inner ear to treat hearing loss. But one of the most difficult challenges they face is measuring the concentration of candidate drugs to insure that they have reached the intended delivery site within inner ear during pre-clinical trials.
“If you can’t be sure of the amount of drug reaching the cochlea—the auditory portion of the inner ear targeted by new drugs—you can’t be sure that the candidate drug is responsible for changes in hearing during treatment,” said Jeff Borenstein, principal investigator for the intracochlear drug delivery device at Draper Laboratory. “Our device is capable of clearly showing whether the desired concentration of the drug is reaching the inner ear,” an essential step toward gaining FDA approval.
Draper is collaborating on an NIH-funded project with researchers at the Massachusetts Eye and Ear Infirmary. A key component of the device developed by the team—the micropump that delivers drugs directly to the cochlea—has worked successfully during numerous pre-clinical trials, demonstrating its functionality while meeting challenging power and size requirements required for extended use in future ambulatory animal models, and ultimately in human clinical applications. The device also enables on-board sample acquisition from the cochlea for precise pharmacokinetic studies and measurements of drug concentration. The micropump is driven by a series of custom-designed microactuators that enable low power operation.
The results of testing were published Feb. 17 in the journal Biomedical Microdevices. In parallel with the demonstration of a pre-clinical device, development is proceeding to use Draper’s device for implantation in humans for safe and effective local drug delivery treatment of hearing loss, and it could be ready for clinical trials in three years.
Unlike current and emerging approaches, which deliver drugs to the middle ear by requiring repeat injections and depend on indirect transport to the inner ear, Draper’s device delivers precise quantities of one or more drugs in an automated, timed sequence directly into the fluid of the inner ear. A unique aspect of Draper’s technology is the use of a reciprocating delivery cycle that keeps the volume of inner ear fluid constant while mixing in the drug, which is expected to increase both the safety and efficacy of the treatment. Draper’s method eliminates many of the issues that can keep a patient from staying the course with treatment, including the toxic side effects associated with systemic delivery and frequent office visits required for other approaches to local delivery for treating hearing loss.
The project is funded by the National Institutes of Health (NIH) through its National Institute on Deafness and Other Communication Disorders (NIDCD) under grant #5 R01 DC006848-08.
Released February 17, 2015