Using Precision Engineering to Streamline Manufacturing of Next-Generation Cellular Therapies
CAMBRIDGE, MA—A promising new treatment for cancer patients has received approval from the Centers for Medicare and Medicaid Services, paving the way for more patients to get the treatment known as CAR T-cell therapy. While this decision will likely expand access, particularly for some lymphoma and leukemia patients, treatment remains prohibitively expensive, prompting some patients to opt for experimental rather than proven therapies.
Why the high cost? “We call it a bioprocessing bottleneck, and it threatens to hinder both patient access to these life-saving therapies and slow advancement of many other therapies,” said Jenna Balestrini, Ph.D., Head of Precision Medicine and Cell Bioprocessing at Draper.
Balestrini works in Draper’s Biomedical Solutions group, which develops technologies to enable automated cellular therapy manufacturing, like those for CAR T-cell therapy. “Current methods for modifying genes for cellular therapies are expensive, time consuming to use, difficult to scale and limited in the kinds of genetic material that can be delivered,” she said. “We believe we as scientists can do better.”
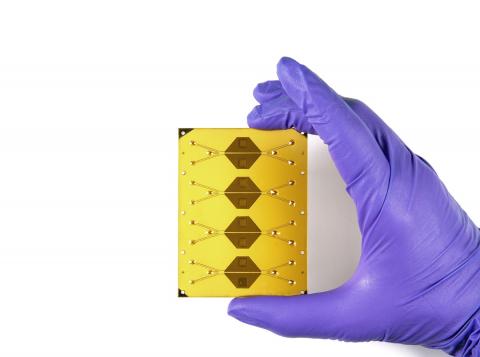
Draper’s Vishal Tandon, Ph.D., agrees. Tandon is leading development of new devices for gene modification that use electroporation instead of viral vectors to deliver genes to a patient’s cells. “Our team has designed a microfluidic electroporation platform that enables precise, controlled gene delivery in T-cells and is capable of processing billions and potentially trillions of cells in about an hour.”
Draper recently tested the system and published the results in the journal Lab on a Chip.
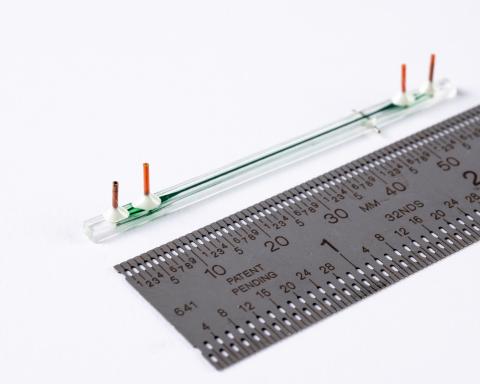
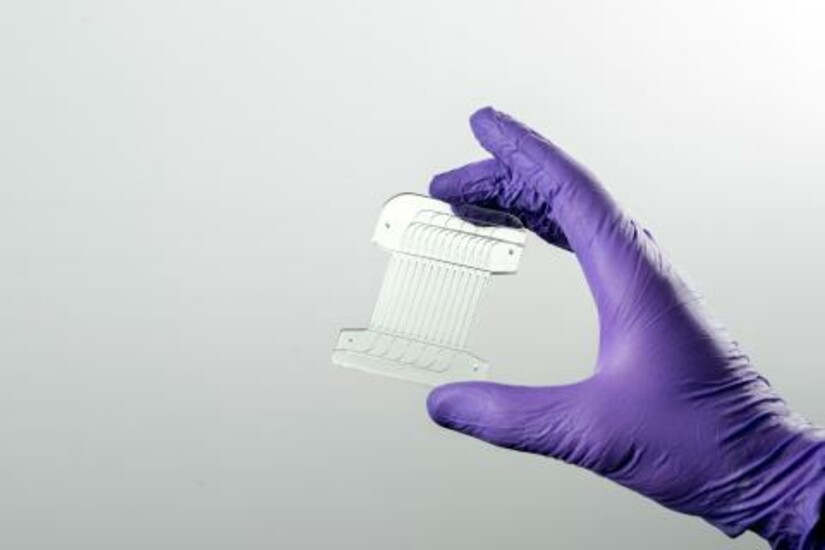
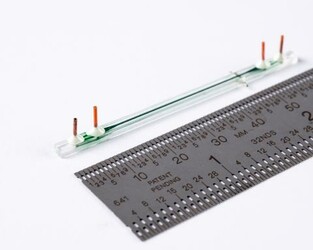
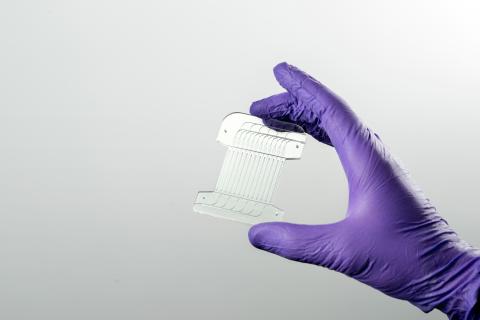
The paper describes a proof-of-concept microfluidic system that combines two unit operations used in cell-therapy manufacturing in an automatic, continuous-processing format. The first unit exchanges cells from growth media into electroporation media using acoustophoresis induced by ultrasonic excitation, a process normally requiring centrifugation. The second unit delivers genes via continuous-flow electrotransfection.
Draper tested the system on human T cells (the type of cell used for CAR T-cell therapy) by automatically moving them from growth media to electroporation media, and then transfecting them with genetic material in the form of mRNA. The authors achieved a continuous media exchange, while maintaining high transfection efficiency with almost no negative impact on cell viability. In recent applications, Draper’s electroporation modules have delivered more than 90% mRNA transfection efficiency without reducing viability and demonstrated delivery of other payloads, including RNPs, pDNA and proteins in both resting and activated T cells.
The systems are fabricated from hard plastic substrates, supporting a path toward large-scale manufacturing of disposable units for clinical use.
The paper’s authors, all from Draper, are Peter Hsi, Rebecca J. Christianson, Ryan A. Dubay, Charles A. Lissandrello, Balestrini and Tandon.
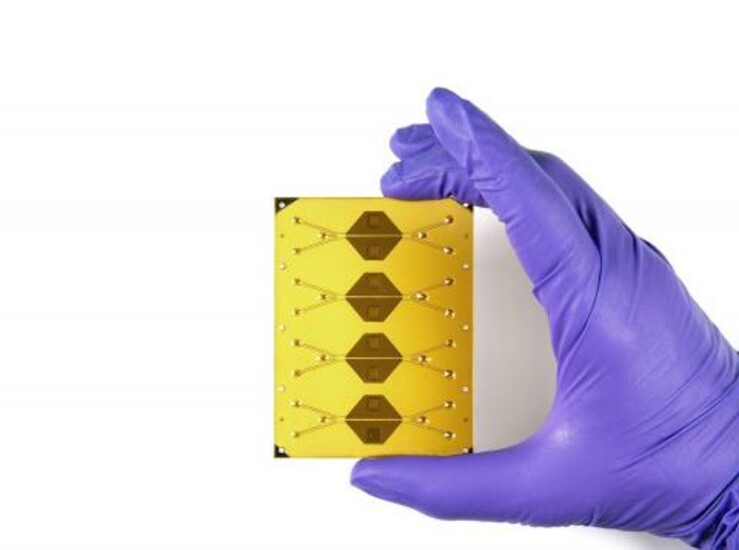
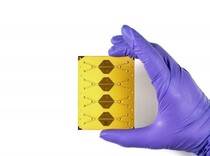
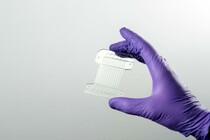
The system is part of an integrated portfolio of resources at Draper intended to help produce next-generation cell therapies and increase patient access to these solutions. The portfolio includes Draper’s Acoustic Enrichment Module, a microfluidic system that offers precision control of flow to optimize purity of lymphocytes collected; Draper’s Microfluidic Electroporation Module, a system that improves transfection efficiency and control without compromising cell viability or throughput; and Draper’s Microfluidic Transduction Module, which can effectively transduce higher numbers of cells by colocalizing cells with vector.
Released August 28, 2019